Wong claims that the procedure is evaluated for filling a monoclonal antibody and in exploratory scientific tests for vaccines. He claims that a number of packages, starting from early- to late-stage medical, are making use of the process.
Kram adds that there are two Most important techniques to continue to keep the item cool although utilizing BFS technology. “The first does probably the most work, keeping the product or service at a small temperature (e.
It's important to obtain correct expertise in regards to the solution’s warmth sensitivity. The BFS approach does impart Vitality in the product or service but in a method which can be controlled and dissipated in a short time. Maintaining the product or service perfectly under an outlined upper temperature and bringing it back to room or maybe a decreased temperature in just a limited period is really a uncomplicated approach that can be defined and examined.
To even further the information and understanding of the process controls,Catalent, in collaboration with Air Dispersions Ltd., ran numerous experiments microbially difficult the system to identify vital Handle parameters and supply the industry with knowledge necessary to support this manufacturing process3.
Containment - The containment of the products is the most fundamental perform of packaging for medicinal items. The look of high-high quality packaging need to take into account each the needs from the products and from the manufacturing and distribution method.
NovaCina’s blow-fill-seal technology offers several different volumes and displays enabling our clients to bring revolutionary supply systems to sector.
The meetings are usually held in beautiful or intriguing places which contributes into the exclusive atmosphere and camaraderie at these meetings.
With BFS, the reduction in container body weight is beneficial from a logistical standpoint, though a discount in contamination and particulates—for the reason that filling and closure take place directly—is a price-add for top quality.
Unither’s Blow-Fill-Seal teams tackle the technology transfer of items made by our customers or by 3rd events. They may also handle the whole growth of customised medicine or healthcare equipment.
The BFS system is sterilised in situ as well as sterile boundary check here will not be breached, just about eliminating the pitfalls related to human intervention.
Although equally filling approaches can operate at speeds of around three hundred to four hundred containers/min, usually there are some parameter dissimilarities to notice. With BFS, the container is plastic as an alternative to glass, plus the relatively very small important zone is put in inside the machine.
Tracing its origins supplies Perception into how this innovation has advanced to satisfy stringent sterility needs.
The technology can then be leveraged For brand spanking new markets, and change just how a product is sent to the affected individual. It truly is evident in the creation of latest container closures that fulfill certain affected individual here requirements, like closures with multiple ports, or simply a flat design and style that fits in just a more compact shipping and delivery product. Ultimately, the technology facilitates container layouts which will deliver goods far more properly.
When compared with the laborious, multi-move process for standard glass vial filling, BFS technology kinds, fills and seals the principal sterile container, generally in fewer than 15 seconds. The aseptic filling machine efficiently acts like an isolator and incorporates the Class A filling disorders inside of its footprint, minimizing the level of managed House necessary and the number of procedure variables associated.
 Jake Lloyd Then & Now!
Jake Lloyd Then & Now!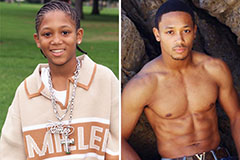 Romeo Miller Then & Now!
Romeo Miller Then & Now! Jennifer Love Hewitt Then & Now!
Jennifer Love Hewitt Then & Now! Joshua Jackson Then & Now!
Joshua Jackson Then & Now! Sydney Simpson Then & Now!
Sydney Simpson Then & Now!